- Isotopes Of The Same Element Have The Same Number Of Protons
- Humm, An Opinion Of One Man Or Woman. I Of Path Do Not Believe It In Any Respect, Though I've Continually Been Just A Little Suspect Over The Ethic..
- This Site Might Help You. RE: Isotopes Of The Same Element Always Have The Same ? AMU Number. \r A-number. \r Z-number.
A family of people often consists of related but not identical individuals. Elements have families as well, known as isotopes. Isotopes are members of a family of an element that all have the same number of protons but different numbers of neutrons.
The number of protons in a nucleus determines the element’s atomic number on the Periodic Table. For example, carbon has six protons and is atomic number 6. Carbon occurs naturally in three isotopes: carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes.
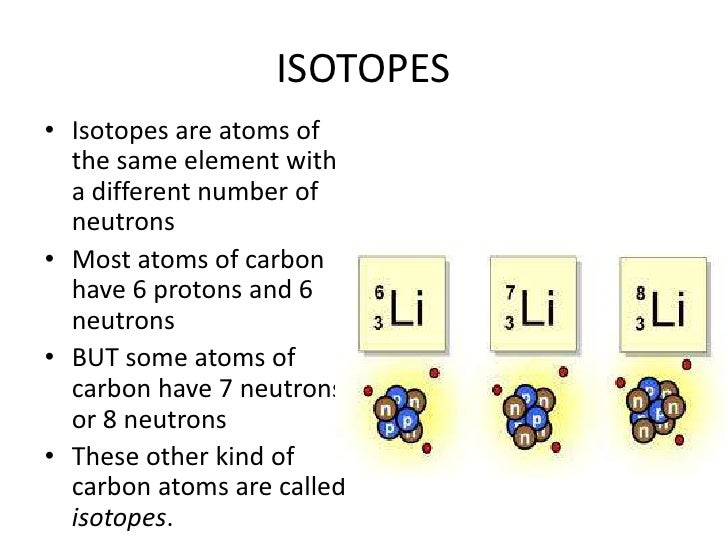
The addition of even one neutron can dramatically change an isotope’s properties. Carbon-12 is stable, meaning it never undergoes radioactive decay. Carbon-14 is unstable and undergoes radioactive decay with a half-life of about 5,730 years (meaning that half of the material will be gone after 5,730 years). This decay means the amount of carbon-14 in an object serves as a clock, showing the object’s age in a process called “carbon dating.”
Isotopes of a given element have the same number of, but different numbers of in their nucleus. Aug 17, 2012 The definition of the isotopes of a given element is that they have the same number of protons but different numbers of neutrons. From the definition, you can conclude that the answer is not 2. Also, since the number of protons determine their location in the periodic table, they must also have the same location in the periodic table, so it's. Isotopes are atoms that contain the same number of protons, but they have different number of neutrons. Drivers pixela mobile phones & portable devices. This also could be understood as isotopes are those atoms which have the same proton number (atomic number) but a different nucleon (mass) number. Note: all atoms that are of the same element contain the same number of Protons. The atoms of an element which have the same number of protons and different number of neutrons are called (a) isotopes (b) isobars (c) isotones (d) isomers MCQ on Chapter: Isotopes, Isobars and Isotones - Read Chemistry.

Isotopes have unique properties, and these properties make them useful in diagnostics and treatment applications. They are important in nuclear medicine, oil and gas exploration, basic research, and national security.
DOE Office of Science & Isotopes
Isotopes Of The Same Element Have The Same Number Of Protons
Isotopes are needed for research, commerce, medical diagnostics and treatment, and national security. However, isotopes are not always available in sufficient quantities or at reasonable prices. The DOE Isotope Program addresses this need. The program produces and distributes radioactive and stable isotopes that are in short supply, including byproducts, surplus materials, and related isotope services. The program also maintains the infrastructure required to produce and supply priority isotope products and related services. Finally, it conducts research and development on new and improved isotope production and processing techniques.
Isotope Facts
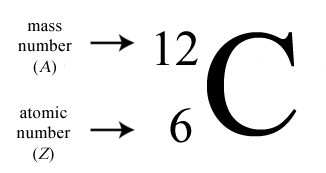
Humm, An Opinion Of One Man Or Woman. I Of Path Do Not Believe It In Any Respect, Though I've Continually Been Just A Little Suspect Over The Ethic..

_0.jpg)
- All elements have isotopes.
- There are two main types of isotopes: stable and unstable (radioactive).
- There are 254 known stable isotopes.
- All artificial (lab-made) isotopes are unstable and therefore radioactive; scientists call them radioisotopes.
- Some elements can only exist in an unstable form (for example, uranium).
- Hydrogen is the only element whose isotopes have unique names: deuterium for hydrogen with one neutron and tritium for hydrogen with two neutrons.
This Site Might Help You. RE: Isotopes Of The Same Element Always Have The Same ? AMU Number. \r A-number. \r Z-number.
Resources and Related Terms
- National Isotope Development Center (Isotope Basics)
Scientific terms can be confusing. DOE Explains offers straightforward explanations of key words and concepts in fundamental science. It also describes how these concepts apply to the work that the Department of Energy’s Office of Science conducts as it helps the United States excel in research across the scientific spectrum.
