Graphene FET chip (GFET-S20 chip) is a graphene based field effect transistor which can be coated on silicon substrate by atmospheric pressure chemical vapor deposition (CVD). It also has similar gate insulators in the source and drain. Part 1: Introduction. LayoutEditor tutorial part 1 shows provides an introduction to layout out graphene FETs on a purchased graphene wafer. The introduction provide background on the wafers, tools, and design rules used in the layout tutorials. This is part of a series of tutorials that shows how to construct graphene FETs and test structures on a purchased graphene wafer.
What are the properties of Fullerenes? We can start with their simple description. Fullerene is a molecule of Carbon. The shape of the molecule can be in the form of a hollow sphere, ellipsoid, tube, and many other shapes.
The first fullerene molecule was produced in 1985 by these scientists; Richard Smalley, Robert Curl, James Heath, Sean O'Brien and Harold Kroto at Rice University. It is important to mention that Kroto, Smalley and Curl were awarded the Nobel Prize in Chemistry in 1996, for their roles in the breakthrough discovery of this class of molecules.
In the early 2000s, the properties of fullerenes were passionately researched. They considered that fullerenes could be used in armors and just a few years later, they discussed its potential use in medicine. Another possible use was to be a light-activated antimicrobial agent. The scientists also studied their conductivity and heat resistance.
It was clear that fullerenes were very promising nanomaterials with more discoveries yet to come.
'Carbon has this genius of making a chemically stable, two-dimensional, one-atom-thick membrane in a three-dimensional world. And that, I believe, is going to be very important in the future of chemistry and technology in general.' Richard Smalley
Properties of Fullerenes and Their Types
Fullerene molecule has unique chemical, physical and physico-chemical properties. The molecule is three-dimensional and spherical and its properties can be described as follows:
- It was proved that the molecule can act as a semiconductor, conductor and under specific conditions as superconductor.
- They can display the photochromic effect. What is the photochromic effect? Trivially, it is a change in light transmission based on the intensity.
- Fullerenes are relatively safe and inert, but despite this fact, they can create active derivatives.
- They also have the ability to form compounds with many different sorts of material. This includes the ability to absorb other substances inside the molecule and also free radicals
These characteristics differ as it depends on the materials with fullerenes or fulleroid fragments. Therefore fullerenes are multi-purpose and can be used in many applications.
Types of Fullerenes
Fullerenes have several structural variations. Such as:
- Buckyballs clusters: The most common is C60 and the smallest one is C20 (It is the unsaturated version of dodecahedron.).
- Nanotubes: They are either single or multi-walled. These fullerenes (hollow tubes) have very small dimensions.
- Megatubes: As their name suggests, they are larger in dimensions than nanotubes and they are prepared with the walls of different thickness.
- Polymers: Two and three dimensional polymers are prepared under high pressure and high temperature. The Atom Transfer Radical Addition Polymerization (ATRAP) route is used to form single-stand polymers.
- Nano onions: These are spherical particles which are based on multiple carbon layers surrounded a buckyball core.
- Linked 'ball-and-chain' dimers: Two buckyballs are linked by a carbon chain.
- Fullerene rings
Let's Have a Closer Look at Buckminsterfullerene Properties
The smallest fullerene molecule that contains pentagonal and hexagonal rings and no two pentagons share the same edge. This is the description of Buckminsterfullerene. They are also very common in terms of natural occurrence. It is often found in soot.
As it was mentioned before C60 was discovered in 1985, by a group of determined scientists and three of them were later awarded by the Nobel Prize in Chemistry, in 1996. Kroto, Smalley and Curl made a major breakthrough discovery. Who are they and what they achieved?
Richard Errett Smalley (1943 – 2005)
Richard Smalley was the Professor of Chemistry and also the Professor of Physics and Astronomy at Rice University, in Huston, Texas. He was an advocate of nanotechnology and its applications. In the late 1990s he advocated for the need for cheap, the clean energy as he believed this would be number one problem in the 21th century. Very interesting is his list 'Top Ten Problems of the Humanity for Next 50 Years'.
Except the most prestigious Nobel Prize in Chemistry (1996), he also obtained Irving Langmuir Award (1991) and E.O. Lawrence Award (1991). His lifetime work was also recognized by the US Senate as they passed the resolution to credit him a title 'Father of Nanotechnology'.
Sir Harold Walter Kroto (1939 – 2016)
He was a British chemist. He received many honors and awards through his life including the Noble Prize that he shared with Smalley and Curl for discovering the fullerenes. He was very productive scientist and he held many positions in academia through his career. Kroto was Professor of Chemistry at Florida State University, but he spent the largest part of his career at the University in Sussex. He promoted the science education and was known for his critical approach towards religious faith. He obtained FRS (1990), Nobel Prize in Chemistry (1996), Knight Bachelor (1996), and Michael Faraday Prize (2001).
Last but not least.
Robert Floyd Curl Jr. (1933)
He is a University Professor Emeritus, Pitzer-Schlumberger Professor of natural Sciences Emeritus and Professor of Chemistry Emeritus at Rice University. He is one of the trio scientists who were awarded Nobel Prize in Chemistry. During his career he was very productive and was honored and awarded many times. For example he obtained his first award in 1957, Clayton Prize, Institute for Mechanical Engineers and he got his latest one in 2015, Citation of Chemical Breakthrough Award. His later researches involved physical chemistry, DNA genotyping and sequencing instrumentation, creating photoacoustic sensors for trace gases using quantum cascade lasers.
Their greatest discovery, Buckminsterfullerene (C60) is named after Richard Buckminster Fuller. He was an architect, designer and inventor, who popularized the geodesic dome. They thought this name is perfect as the Buckminsterfullerene has a similar shape.
It is a type of the fullerene with the formula C60. We could compare his structure to the soccer ball (football). It is made of 20 hexagons and 12 pentagons where a carbon is located at each vertex of each polygon and a bond along each polygon edge.
This fullerene is the most common fullerene that occurs naturally, for example in small quantities in the soot. Very interesting is the fact that the compound was detected in deep space.
Production Method
Fullerenes are produced by sending a large current between two nearby graphite electrodes in an inert atmosphere. When carbon plasma arc between the electrodes cools into sooty residue, then many fullerenes can be isolated.
Generally, they are dissolved in hydrocarbon or halogenated hydrocarbon and separated by using alumina columns.
C60 Fullerene Properties – The Fullerene Chemistry Was Born
Since its discovery in 1985 till 1990 they were series of studies which indicated that C60 and also C70 are exceptionally stable. These studies also provided satisfying evidence of the cage structure proposal.
Buckminsterfullerene is a molecule that undergoes a wide range of novel chemical reactions. Its chemical and physical properties can be used in many possible applications.

For example C60 has an ability to accept and donate electrons, this is a behavior that can be used in batteries and advanced electronic devices. Its compound is very stable and can resist high temperatures and pressures. Another promising ability is that the exposed surface of the structure can selectively react with the other species and its spherical geometry remains the same.
The molecule can also add the atoms of hydrogen and of the halogen elements and the halogen elements can be replaced by other groups, for instance phenyl. Therefor there are more possible routes to a wide range of fullerene derivatives. Some of them show advanced materials behavior. If we speak about superconductivity, particularly important are crystalline compounds of C60 with alkali metals and alkaline earth metals. Only these molecular systems are superconductive at relatively high temperature.
Nanowire Fet
Possible Applications:
- Research and development – This fullerene is very promising especially in Biomedicine research. It can be used for biomedical engineering, especially in cancer treatment research. The scientists would like to use C60 fullerene in photochemotherapy, which would be a good alternative to radiation therapy. What is photochemotherapy? After being absorbed by the cancer cells, fullerene could turn oxygen inside the cancer cell into radical oxygen. This would be achieved by the light radiation in order to evoke the apoptosis, which means that the radical oxygen would destroy all important biomolecules in the cell, causing that much damage that the cell would not survive. They show very good biocompatibility which predicts their bright future in other biochemical applications.
- Automotive industry – Fullerenes could be used as electro catalysts. They could lower the pollution caused by the fossil fuels.
- Coating and paintwork materials – They can enhance the properties of the coating agents.
- Cosmetics – Fullerenes are excellent antioxidants, they can react with many radicals and work as a shield against them. This ability is also used in cosmetology. Some creams contain several fullerene derivatives, very common is polyethylene glycol modified fullerene C60.
- Electronics
- Composite and polymeric materials
- Powder metallurgy
- Lubricants
Physical Properties:
| Chemical formula | C60 |
| Molar mass | 720.66 g·mol−1 |
| Appearance | Dark needle-like crystals |
| Density | 1.65 g/cm3 |
| Melting point | sublimates at ~ 600 °C (1,112 °F; 873 K)[1] |
| Solubility in water | insoluble in water |
Types and Properties of Buckyballs
We already know that the Buckminsterfullerene is one of the buckyballs fullerenes. Another buckyballs representative which was predicted and discovered in 2007 is Boron buckyball.
Boron Buckyball
This fullerene uses boron atoms instead of the usual carbon. The B80 structure suggests being more stable than C60. Each of its atom form 5 or 6 bonds. In fact the B80 structure is more similar to the geodesic dome which was popularized by Buckminster Fuller. It doesn't form hexagons like C60, it uses triangles instead. However pure Boron fullerenes are unlikely to exist in nature.
Other Buckyballs
C70 is also quite common fullerene. There are also fullerenes C72, C76, C84 up to 100 carbon atoms. The smallest fullerene is dodecahedron C20. There was also discovered class of novel molecules which consists of 80 carbon atoms. These fullerenes, trimetaspheres, have a great potential to be used in diagnostics, therapeutics and in organic solar cells.
Physical and Chemical Properties of Fullerenes
Except the buckyballs there are other not less interesting fullerenes types, such as carbon nanotubes, mega tubes, polymers or Nano onions. All of them have unique chemical and physical properties that are already successfully used in many applications or they are being researched and tested.
Carbon Nanotubes
This fullerene type was discovered by Japanese scientist Iijima Sumio of NEC Corporation's Fundamental Research Laboratory, Tsukuba Science City in Japan. In 1991 he investigated material that was extracted from solids. These solids grew on the tips of the carbon electrode, this happened after they had been discharged under C60 formation conditions. Sumio realized that the solids consisted of tiny tubes.
Nanotubes can be described as cylindrical fullerenes. They are not very wide, usually just a few nanometers, but they can range in length from just a few micrometers to several millimeters. This unique structure is the secret of their extraordinary macroscopic properties, such as high tensile strength, high electrical conductivity, high ductility, relative chemical inactivity and high resistance to heat.
Nanotubes are characterized as single-walled nanotubes and multi-walled nanotubes.
Single-walled Nanotubes characteristics:
Their electrical conductivity can show metallic or semi conductive behavior. They could likely be used in miniaturizing electronics. They could also be excellent conductors. There is also one useful application of single-walled nanotubes in the development of the first intermolecular field-effect transistors (FET). These fullerenes are predicted to make a large impact in the electronics in 2020, which is very near future indeed.
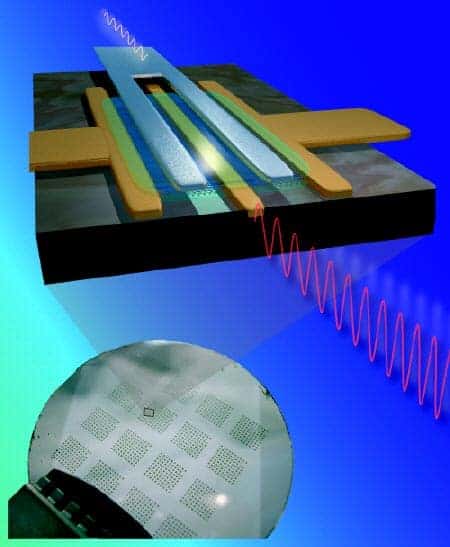
Multi-walled Nanotubes characteristic:
Multi-walled carbon nanotubes consist of multiple rolled layers of graphene. The space between the layers is 0.34 nm. This is exactly the same distance as between the crystal graphite layers. There are two models that can be used to describe the structure of the multi-walled nanotubes, which is Russian Doll model and the Parchment model. The first named model is observed more commonly.
Due to their telescopic motion ability of the inner shells and their extraordinary mechanical properties, multi-walled nanotubes have a great potential to be used as a main component in the future Nano mechanical devices.
They also exhibit faster phonon transport than diamond. Before carbon nanotubes were discovered, diamond was recognized as the best thermal conductor. Their electric current-carrying capacity is approximately four orders of magnitude higher than that of copper, very impressive results.
Carbon nanotubes are undoubtedly exceptional materials and their chemical and physical properties allow them to modify any composite material. They improve the quality and also the durability of the end products. They are strong and flexible and as it was mentioned before they are great heat and electric conductor. Carbon nanotubes have also very promising optical properties for instance the absorbance of fluorescence.
Carbon nanotubes can be used in these application areas:
- Composite material modification / master batches
- Components for electronics
- Chemical industry
- Oil-refining industry
- Building industry
- Coatings – they are versatile coating materials.
- Lacquer and paints
- Ceramics
- Concrete
- Energy – supercapacitors as they are not only great conductors, but also have very big surface area. Also solar cells, fuel cells, Li-ion batteries (for notebooks and mobile phones) and hydrogen storage.
- Polymer materials – thermoplastic and thermosetting plastic
- Ecology – Photovoltaic technologies, multiuse absorbents, multiuse filters (water purification)
- Bioengineering – Very exciting and hopeful is the cancer research, drug and gene delivery, cell tracking and labelling, biosensors ( they could be used for example in medicine, food technology or military), synthetic implants, receptors in viv
Their strength and flexibility can be used in controlling other nanoscale structures securing their important role in the nanotechnology engineering.
Megatubes
Megatubes are basically extremely large carbon tubes; some can even exceed 5 μm in diameter. They can be produced either by laser or electric arc techniques by using graphite, transition metal catalyst, and a reactive third body gas.
In fact they are large enough to be observed by the optical microscope. The scientists also believe that they reported a synthesis of the first self-assembled branched nanotubes. X-ray photoelectron spectroscopy has proved that these tubes also contain significant amount of nitrogen atom, which is incorporated into the graphite lattice. Later on it was shown that these nitrogen functionalities interacted with rhenium pentacarbonyl bromide where it serves as anchor points to tether molecules to the surface of the tubules.
Polymers
There are two possible ways how to prepare various kinds of polymeric fullerenes, either by using functional polymers to react with fullerenes or by synthesizing polymers in the presence of fullerenes. This way is prepared side-chain polymers, fullerene and capped polymers and so on.
If polymers are combined also with carbon nanotubes, the outcome material has chemical and physical properties which make it a great candidate for many applications such as: data storage media, photovoltaic cells and photodiodes, photosensitive drums for printers and optical limiting devices.
Nano Onions
Nano onions are currently one of the most fascinating carbon forms. They are being researched widely in biological cell imagining as they are bio safe and non-toxic. Their functionalization with different functional groups improves the solubility and biocompatibility. It increases the ability to penetrate into the cells. Due to their small size and high surface area they can conjugate different diagnostic and therapeutic agents. It can lead to new theranostic applications.
What Are the Properties of Fullerenes – The Summary
We have already learned that fullerenes have truly unique chemical and physical properties, which have great potential in many application areas the same as they are very promising agents in cancer treatment.
Graphene Transistors
The discovery of Buckminsterfullerene (C60) in 1985 was a breakthrough that led to a discovery of many more fullerene types. Since then the scientists have not stopped the research of their properties and possible use. In fact in the past 10 years, over 1,500 worldwide patents have been filed for their production and applications. Fullerenes are wanted across the fields and industries as they are multi-purpose materials.
Fullerenes are already used in coatings, paintwork materials, cosmetology (due to their antioxidant properties), electronics, and lubricants, composite and polymeric materials. There are also ongoing numerous studies, researches and tests to discover their full potential.
Graphene Fet
As Richard Smalley, Laureate of the Nobel Prize in Chemistry 1996, said: 'The more we understand what happens in the living cells, the more incredibly powerful you realize things can be when they work from the bottom up, by interaction of one molecule to another.'
Graphene Fet Dna
